Nobel Pursuit

Canada’s first Nobel Prize was won in 1908 by a young Cambridge-trained New Zealander who spent only nine years in Canada and had already left the country by the time the award was announced. But in that short Canadian interlude spent at McGill University, Ernest Rutherford kickstarted a worldwide scientific revolution.
Among his collaborators were two researchers six years his junior: Frederick Soddy, a recent Oxford graduate who was working as a chemistry demonstrator at McGill, and Harriet Brooks, a Canadian graduate student who became the first woman ever to obtain a master’s degree in physics at McGill. His financial benefactor was William Macdonald, an eccentric Montreal tobacco millionaire who abhorred smoking. It was an odd but powerful combination of people.
Rutherford was just twenty-seven years old when, in 1898, he was hired as Macdonald Professor of Physics at McGill. This was an impressive title for one so young, but, even so, he came to Montreal with misgivings: He was an ambitious researcher, and Canada was then on the distant periphery of the scientific world. He was doubtful of what he might be able to accomplish so far from the hub of scientific activity, which was then in England and Western Europe.
He need not have worried. He arrived to find the newest and best-equipped physics lab in the world, and before long he had created his own scientific hub. When Rutherford began his work, the bewildering phenomenon of radioactivity had only recently been observed by scientists in France, and its origins remained a mystery. At the time, scientists believed that atoms were hard kernels that could neither be dismantled nor changed. Their theory held that each element had its own characteristic atom — gold was made up of gold atoms, oxygen was made up of oxygen atoms, and so on — and that the atoms of one element were completely different from the atoms of every other element.
How could this picture be reconciled with the observation of radioactivity? If an atom was irreducible and unchangeable, how could it spontaneously burst into action and emit energy in the form of radiation yet not be reduced and changed in some way?
Using a series of simple but ingenious experiments, and astonishing insight, Rutherford came up with an explanation for radioactivity that fundamentally changed our understanding of the atom itself. But the story of how this brilliant young man was lured to Canada and allowed to flourish here really begins three decades earlier when William Macdonald decided to buy a house.
With 7 uniquely curated newsletters to choose from, we have something for everyone.
Macdonald was a wealthy businessman of Scottish stock who had been born on Prince Edward Island in 1831 and made his fortune in Montreal, where he processed imported tobacco leaves into products sold for chewing or pipe smoking. He got his start during the American Civil War by buying leaf from the South and selling product to the North, when the two adversaries could not deal with one another directly; but he continued to expand his empire after the war ended.
As a bachelor obsessively focused on his work, he boarded in hotels until he was nearly forty years old before deciding to move into a permanent home in an upscale Montreal neighbourhood with his mother and unmarried sister. His new house turned out to be near McGill College — as it then was known — and the home of its principal, John William Dawson. The two men became friends.
Macdonald was a man of contradictions. He was ultimately very rich — becoming one of the wealthiest men in Canada — yet he lived frugally and dressed like a pauper. He made his money from tobacco, yet he detested smoking and considered smokers to be stupid for indulging in their habit. And in his business practice, as in his life, he pinched pennies wherever he could, yet when he finally turned to philanthropy he was generous to an extreme.
Even though his business was a huge financial success, Macdonald once admitted that he was not proud of it, because of what it produced. He himself suggested that shame might have motivated the donations he made in his later years. Whatever the reason, after he turned forty he increasingly shifted his focus to philanthropy, and, with the encouragement of his friend Dawson, McGill became his greatest beneficiary.
Dawson was principal of McGill for thirty-eight years in all, longer than any person before or since, and he was the first to put the institution on a solid foundation both financially and academically. It was during his tenure that the institution’s name changed from McGill College to McGill University. As a geologist, he was particularly keen on developing the university’s infrastructure for pure and applied sciences. And, over the years, Macdonald was increasingly eager to oblige.
He began with relatively modest gifts, but, by the early 1890s, he had endowed numerous professorships and paid for construction of the Macdonald engineering, chemistry, and physics buildings. His generosity even extended to supplying his buildings with the best available modern equipment for teaching and research.
Before the Macdonald Physics Building opened at the beginning of 1893, its benefactor asked the professor of physics at the time, John Cox, to tour the United States and Europe to find out what equipment a top laboratory should have and to let him know the cost. Cox dutifully did as he was told and came back with an estimate of five thousand British pounds. Macdonald gave him six thousand. He also endowed a second physics professorship, and, as the research program grew, he donated more money for equipment, the final total rising to twenty-two thousand pounds — equivalent to about five million Canadian dollars today.
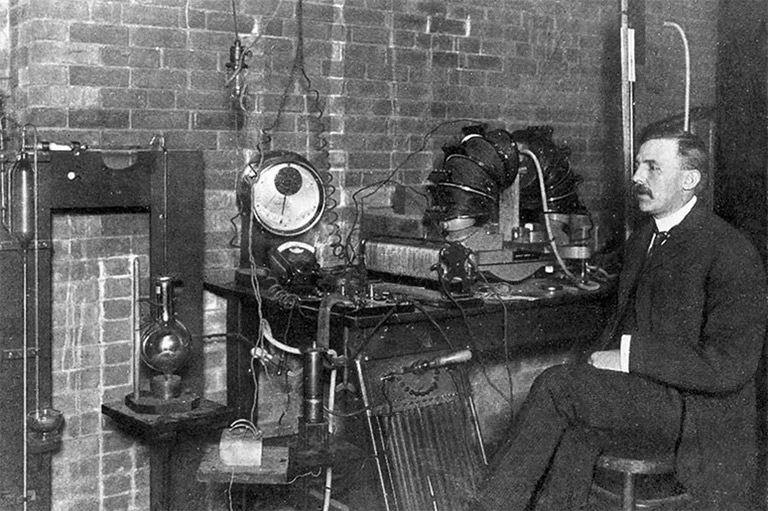
The first man to fill Macdonald’s newly endowed professorship came in 1893 but departed in early 1898, leaving the university with an important vacancy to fill. To find a suitable candidate, a high-level delegation made up of the university’s principal, William Peterson — who had succeeded Dawson three years before — and the head of physics, John Cox, travelled to Britain in the summer to visit the leading physics research centre in the English-speaking world, the Cavendish Laboratory at Cambridge University, where the celebrated J.J. Thomson presided.
This was a time-consuming expedition in those days for two of the most senior members of the university administration. It reflects the priority the university placed on meeting Macdonald’s expectations for generating high-profile results from his generous donations.
When the McGill visitors met with Thomson, he told them without a moment’s hesitation that his student Ernest Rutherford, who was visiting from New Zealand on a scholarship, was their man. He said he had never had another student “with more enthusiasm or ability for original research.” That recommendation was all they needed, and, within a month, Rutherford had been offered, and had accepted, the job. Another month later, on September 18, 1898, he disembarked from the SS Yorkshire in Montreal.
In appearance and behaviour, Rutherford did not fit the popular image of a scientist. He had grown up as the fourth of twelve children in pioneering country on the South Island of New Zealand. His father was a farmer and a wheelwright, his mother a former schoolteacher. He never shed those origins, and, in adulthood, he was occasionally mistaken for a successful farmer rather than a cerebral scientist.
He was a tall man with a strong voice and a hearty laugh. He made friends easily. In his research work, he was described as a “happy warrior,” ebullient in success and optimistically persistent in adversity. Once at McGill, he wasted no time in gearing up to begin his work in the splendid facilities that awaited him there.
But research was not the only thing on his mind. Before leaving New Zealand in 1895 to begin his three-year scholarship in Cambridge, Rutherford had become engaged to nineteen-year-old Mary Newton. Now three years had passed, and he was not heading home to marry his bride but instead had moved on to another location just as remote from New Zealand. He was driven to that choice by his ambition to make a name for himself in research, but he certainly did not intend to abandon his fiancée.
In his first letter to her, sent immediately after he was offered the McGill job, he began with the words: “Rejoice with me, my dear girl, for marriage is looming in the distance.” With a professor’s salary, he told her, he could pay off his education debts and save enough money to be able to marry her in eighteen months.
In fact, his salary appears to have been quite generous, although the young scientist noted that it was “small compared with the endowment of the Laboratories and the enormous money spent on them.” Nevertheless, negotiation was out of the question. As he explained, the millionaire Macdonald who “supplied all the coin and continues to do so” had firm ideas on salary. Rutherford wrote that Macdonald himself “lives on £250 a year, so he reckons a professor should live on £500.” It was a point well taken: A salary of five hundred British pounds was equivalent to more than one hundred thousand dollars in today’s Canadian currency.
With a marriage trip to New Zealand deferred, Rutherford could apply himself to the job at hand, for which the university had set high expectations. In Rutherford’s words, he was “expected to do great things. I am expected to do a lot of original work and to form a research school in order to knock the shine out of the Yankees!”
Advertisement
As soon as he had found a place to live in Montreal, Rutherford ordered supplies of uranium and thorium from the laboratory he had left in Cambridge. Both substances were known sources of radioactivity, and he had already begun making measurements on the former before leaving for Canada. In a paper that was actually published just after he got to McGill, he reported having identified two different types of radioactive emissions from uranium, distinguished by their ability to penetrate thin foils of aluminum. He named the type that was readily stopped “alpha rays” and the type that passed easily through “beta rays” — terms still in use to this day. His initial plan at McGill was to study the radioactivity from thorium to see if it had similar properties.
One of the first graduate students he recruited to his research group was a twenty-two-year-old woman, Harriet Brooks, who had been a star undergraduate at the university. She would ultimately play an important role in his work, but, at first, Rutherford assigned new students like Brooks to more secure projects than the adventuresome subject of radioactivity.
His first short-term collaborator on radioactivity research was R.B. Owens, a young professor of electrical engineering who was casting about for a research project and welcomed Rutherford’s invitation to join him. To their astonishment, they soon observed that the strength of the radiation from thorium was not constant, like that from uranium, but was sensitive to whether the door to the laboratory was open or closed. The only plausible explanation was that some component of the radioactive source was apparently being wafted around by the breeze.
The fog was starting to clear, but the picture that began to emerge was almost too revolutionary to believe.
But what could it be? The thorium itself was a solid, and alpha and beta rays were already known to be insensitive to air movement.
Rutherford dug into this puzzle, working on his own throughout 1899. With carefully designed experiments, he determined that thorium was emitting some kind of vapour or gas, which he named, noncommittally, “thorium emanation.” He managed to convey some of this emanation down a glass tube away from the original thorium, and thus found it to be radioactive itself but with an extraordinary new property: The intensity of its radiation decreased in time, with half of it having disappeared in about a minute. This was very different from thorium radioactivity, which appeared to be inexhaustible.
More startling still was his discovery that the emanation appeared to induce yet another form of radioactivity on any surface with which it came into contact. This activity also decreased in time, but at a very different rate from that of the emanation: It took more than eleven hours to lose half of its intensity.
This made Rutherford the first person ever to observe the time decrease of radioactivity. He was also insightful enough to recognize that the rate of decrease was exponential and that each different activity could be characterized by what we would today call its “half-life,” in other words, the time it takes for it to decrease to half intensity. But what did it all mean?
Save as much as 40% off the cover price! 4 issues per year as low as $29.95. Available in print and digital. Tariff-exempt!
By the spring of 1900 Rutherford had accomplished a lot, but in truth he had uncovered more questions than he had answers. He surely had much to ponder as he set off by train at the end of March heading to San Francisco, where he boarded a boat for the long voyage to New Zealand. At last, after a five-year engagement at a distance, he was able to marry his fiancée, Mary Newton. The couple returned to Montreal just in time for classes to begin in the fall.
After such an extended absence, Rutherford was eager to return to his experiments, but he now realized that his path ahead would require the help of a chemist.
McGill’s senior professor of chemistry showed no interest in the subject, but a twenty-three-year-old chemistry “demonstrator” — a kind of teaching assistant — quickly recognized a good thing when he saw it. An Oxford graduate, Frederick Soddy had been hired by McGill into a junior position only a few days before. He was eager to work on a challenging project and was glad to join an inspiring scientific leader in doing so.
The two men worked together throughout 1901, connecting the pieces of the puzzle as best they could. At the same time, Rutherford had Brooks working on the properties of emanation — in this case, the emanation from radium, another radioactive substance with a similar property. By an indirect method, she determined that the emanation was not a vapour — a gaseous phase of radium — but a true gas, characteristic of a distinctly different element.
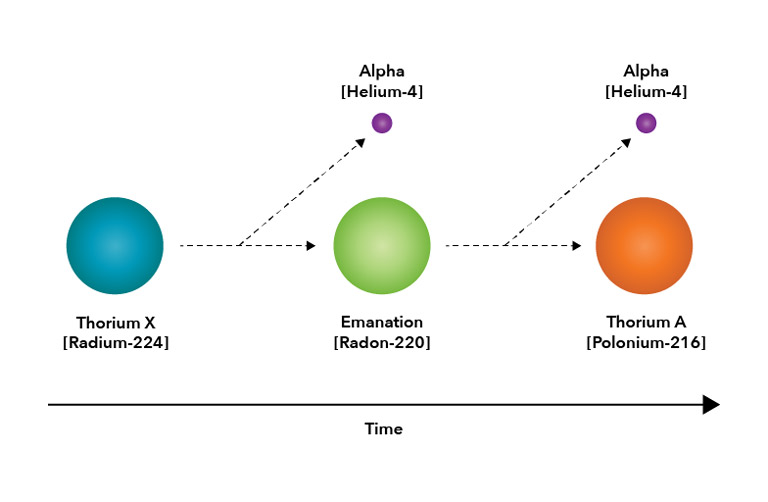
Conclusive proof, though, required chemistry, and early the next year Rutherford and Soddy published their first astonishing results: Thorium emanation was not simply a gas, it was a “noble gas” of the same family as argon — in other words, a gas that does not easily react chemically with any other element. Furthermore, the origin of the emanation, a substance they called “thorium-X,” was chemically different from the thorium source material itself and could therefore be separated from it.
In a second paper published a few months later, they reported having separated thorium-X from its thorium host and having measured its exponential decay to have a half-life of about four days. In addition to that, they found that the thorium host replenished its supply of thorium-X, also exponentially, with half of it being restored in about four days.
The fog was starting to clear, but the picture that began to emerge was almost too revolutionary to believe. Rutherford published thirteen papers in 1902 alone, five of them co-authored with Soddy. Tentatively at the beginning of the year, but with more assurance as the evidence accumulated, Rutherford and Soddy asserted that a primary change in thorium leads to the appearance of thorium-X, which subsequently undergoes a secondary change to produce a gaseous thorium emanation. The emanation, in turn, undergoes another change, leading to the radioactive substance that was found on surrounding surfaces. Each of these substances is chemically different from the others, and each change is accompanied by its own signature radioactivity.
In short, they concluded that radioactivity accompanies a chemical change. To use modern terminology, they found that radioactivity is actually part of the process by which one chemical element can spontaneously decay into another. This was a revolutionary discovery that completely destroyed the concept of an indivisible atom.
In parallel with these studies on the nature of radioactive substances, Rutherford also sought to understand the alpha rays that were ejected by these substances as they decayed. In a brilliantly designed experiment, he showed for the first time that the path of an alpha ray could be bent if it passed through a strong magnetic field. That meant that the “ray” was actually a charged particle of atomic proportions, most likely an atom of hydrogen or helium. (Later, after further study, he proved that it was a helium atom.)
This was the last piece of the puzzle. The only plausible explanation was that the radioactive atom was spontaneously breaking up into two atoms. The smaller of those two atoms — the helium atom — was ejected and then detected as an alpha ray. The second atom — the one that remained behind — was slightly lighter than, and chemically different from, the original atom. The astonishing story was complete, in outline at least!
Advertisement
McGill’s rich benefactor, William Macdonald, kept abreast of Rutherford’s achievements. In fact, he happily purchased specialized equipment that Rutherford requested for his research along the way. He also visited the laboratory periodically to check on progress.
A new arrival to Rutherford’s research group, Arthur Eve, remembered a day in 1903 when Rutherford burst into the basement laboratory to tell his students to open all the windows, put out their pipes, and hide their tobacco; Macdonald was on his way. As Eve put it, “We were all, men and laboratory, dependent on the sale of tobacco, but to use it was a crime!”
By the end of 1903 Rutherford’s discoveries had made him famous, not just among his fellow scientists but also with the general public. That year he was elected to fellowship in the Royal Society of London, the premier scientific organization of the day, and he was showered with invitations to give talks on his work on both sides of the Atlantic.
And Rutherford was a master at it. Not only was the subject of his talks inherently fascinating, but the speaker himself was inspirational. In the words of Rev. John McNaughton, a professor of classics at McGill and not a notable fan of the sciences, “Here was the rarest and most refreshing spectacle — the pure ardour of the chase, a man quite possessed by a noble work and altogether happy in it.”
Before long he began to receive awards, as well as job offers, from the United States and England.
Soddy shared the spotlight. As a mere demonstrator at McGill, he was easily lured back to a higher-profile research position in Britain in the fall of 1903. Rutherford stayed put, however; none of the proffered jobs offered him anything to match the facilities he already enjoyed at McGill.
Rutherford’s position as leader in a new branch of science was secured by the publication of his first book, Radio-activity, in early 1904. It was enthusiastically received and served to define the field for generations of researchers to come, as it went through many successive editions.
In spite of all the attention, he managed to continue his research in 1904 and beyond. Harriet Brooks was back in his lab after two years away, and she was joined by a few new graduate students and a succession of visiting scientists coming to work with the great man himself. Together, these researchers did much to consolidate and to extend what was known of the radioactive process.
Even so, Rutherford became more and more acutely aware of his isolation from the centre of scientific action.
Early on during his time at McGill, he wrote to J.J. Thomson: “Outside the small circle of the laboratory, it is seldom I meet anyone to hear what is being done elsewhere.” This situation was only slightly improved by his occasional visitors and was considerably exacerbated by his very success. For, by now, Rutherford’s team had become immersed in a worldwide frenzy of competition in the field, and his competitors were far closer to the prominent journal publishers in Europe than he was.
His isolation began to rankle him. It was hard to stay ahead of the competition with the Atlantic Ocean adding an extra delay to his communications back and forth with publishers and other researchers. So it was that in late 1906 Rutherford received a job offer that was impossible for him to refuse. The University of Manchester had a physics laboratory as good as the Macdonald Physics Building and with a larger staff; as well, the university had a more abundant source of high-quality research students than McGill could offer. Above all, Manchester was located in the midst of the action. He summed up the job’s attractions in a letter to his mother: “I shall receive a better salary and be director of the laboratory and what is most important to me, will be nearer the centre of things scientifically.”
Rutherford’s impending departure from McGill was greeted by his colleagues with a mixture of regret and warmth. A resolution from the whole Faculty of Applied Science acclaimed his research work as having “extended the fame of McGill University to all parts of the world.” It went on to praise his work in organizing the university courses for higher degrees, stating that “his sincerity and geniality enabled him to take a strong line without risk to the warm personal friendship with every member of the staff.” While the faculty members expressed their regret at his departure, they also rejoiced that he was advancing “to a sphere where he may render still more eminent service to the cause of Science at large.”
And render it he did. For twelve years at Manchester University, and then for eighteen more after that as Director of Cambridge University’s Cavendish Laboratory, Rutherford remained extraordinarily productive. Perhaps most significantly, in 1911 he discovered the structure of the atom, demonstrating that it was made up of a tiny, dense core — the nucleus — surrounded by wispy clouds of electrons. He made other startling discoveries as the century advanced.
For his part, William Macdonald lived another ten years after Rutherford left Montreal and continued his generosity to McGill University. Most notably he was responsible for setting up and endowing Macdonald College at Sainte-Anne-de-Bellevue, McGill’s campus for agricultural studies. In his own life, he was frugal to the end. For a few years he bought eggs from the poultry department of Macdonald College, until one day he told them he would have to stop doing so. “I can get them for two cents a dozen less in Montreal.”
It was not long after Rutherford’s departure from McGill that his work there was recognized with a Nobel Prize. In December of the next year, he and Mary travelled to Stockholm, where he was presented with the 1908 Nobel Prize in Chemistry. The prize citation made clear that it was “for his investigations into the disintegration of the elements, and the chemistry of radioactive substances,” the subjects of his discoveries at McGill.
Rutherford, a physicist, was somewhat taken aback to be given the prize for chemistry. To the Nobel committees, the fact that he had observed the decay of a chemical element meant that he had been doing chemistry.
Rutherford saw it differently, but, typical of the man, he treated it good-naturedly. In an after-dinner speech at the Nobel banquet, he remarked, “I have dealt with many different transformations with various periods of time, but the quickest that I have met was my own transformation in one moment from a physicist to a chemist.”
Equipped for Success
Ernest Rutherford used these scientific tools to investigate radioactivity, to establish the nature of the alpha rays emitted by radium and thorium, and to formulate his theory of radioactive transformation. The items are held in the Rutherford Museum collection at McGill University in Montreal.

Differential air calorimeter: This apparatus was used to study the heating effect of radium and its emanations. The two vases, immersed in a constant-temperature water bath, were sealed and connected by a U-tube containing the liquid xylene. The right-hand flask contained a radioactive source such as radium, the decay of which heated the air in the vase. The heated air expanded, displacing the xylene in the U-tube. This displacement was calibrated by means of a measured current in a known resistance wire in the left-hand flask. This allowed Rutherford to calculate how much heat was generated by the radioactive decay.

Apparatus for the study of ionization in gases: This apparatus measured the ionization current of dry and moist air, achieved by the introduction into the chamber of alcohol or methyliodide vapours. Radioactive emissions from radium or thorium, deposited on a plate at the bottom of the chamber, caused atoms of the gases within (whether air, alcohol, or methyliodide) to ionize, i.e., to gain or lose electrons, and therefore to become positively or negatively charged. Voltages between parts of the chamber resulted in an electric current, called the ionization current. The strength of the ionization current allowed Rutherford to determine the strength of the ionization produced by the various radioactive elements.

Ionization chamber: This apparatus was used by Rutherford and Harriet Brooks to measure the rate of diffusion of radium emanation in air. The long cylindrical ionization chamber was divided in two by a removable gate (the rectangular object with the T-handle seen at right). With the gate closed, air containing radium emanation was injected into the left-hand side of the chamber. The experimenters then opened the gate and measured the time it took for the emanation to distribute itself evenly throughout the whole chamber. They used this measurement in an effort to determine the molecular weight of the emanation atoms.

Harriet Brooks
Canada’s first woman physicist was forced to choose between marriage and science.
When Harriet Brooks enrolled in 1894, McGill University had opened its doors to women only ten years before, and among the full-time students men outnumbered women sixteen to one.
Brooks was not intimidated. She was the only student in her year to graduate with first-class honours in mathematics and physics. She then joined the laboratory of the charismatic new professor Ernest Rutherford.
Rutherford considered Brooks to be an exceptional researcher. By the time she received her master’s degree in physics in 1901 — the first woman at McGill to do so — she had produced enough original results on radioactivity to generate two important papers, which she co-authored with Rutherford.

She continued her studies at Bryn Mawr College, a women’s college in Pennsylvania, where she won a fellowship to study radioactivity at the Cavendish Laboratory of the University of Cambridge, England, where Rutherford himself had trained. This was a plum opportunity, but, as she explained in a letter to Rutherford — quoted in the biography Harriet Brooks: Pioneer Nuclear Scientist by Marelene and Geoffrey Rayner-Canham — she would have to deal with the “objections of my family ... who will think me wholly out of my mind.” Evidently she overcame their objections, since she spent the next year in England.
In 1903 she returned to Rutherford’s lab and soon proved her mettle by publishing two papers on radioactivity in which she broke new ground. Had she been a man, her research career would likely have been launched. As a woman, her prospects in Canada were slim. So she resigned after a year to take a teaching position at Barnard College, a women’s college in New York City.
Two years later, in 1906, she notified her dean that she would be getting married and asked to retain her teaching position. Though Barnard had granted such a request before, Dean Laura Gill adhered to the most common practice at the time and responded that, once married, Brooks would have to leave Barnard. Brooks protested that she thought it her duty “to my profession and to my sex to show that a woman has a right to the practice of her profession and cannot be condemned to abandon it merely because she marries.” It was to no avail.
In emotional turmoil, Brooks broke off her engagement, resigned her position, and embarked with friends for Europe, where she spent a good part of the next year working in the Paris laboratory of Nobel Prize-winning physicist Marie Curie.
By then, Rutherford was on his way to Manchester and eager to enter Brooks’s name for a fellowship to work with him there. At first she agreed, but then a suitor from Montreal, Frank Pitcher, visited her in London to plead his case. She was not easily persuaded, but ultimately she withdrew her name from the fellowship and the couple was married.
Their marriage was a contented one, their son Paul Pitcher later recalled. But science lost a rising star. Brooks died at the age of fifty-six after a lengthy illness, possibly as a result of her earlier exposure to radiation. —John Hardy
We hope you will help us continue to share fascinating stories about Canada’s past.
We highlight our nation’s diverse past by telling stories that illuminate the people, places, and events that unite us as Canadians, and by making those stories accessible to everyone through our free online content.
Canada’s History is a registered charity that depends on contributions from readers like you to share inspiring and informative stories with students and citizens of all ages — award-winning stories written by Canada’s top historians, authors, journalists, and history enthusiasts.
Any amount helps, or better yet, start a monthly donation today. Your support makes all the difference. Thank you!
Themes associated with this article
Advertisement